

Unlike the production of antibody fragments, for which bacterial systems can be used, tetrameric IgGs with suitable glycosylation patterns require eukaryotic hosts. The growing need for fast and cost-effective antibody development and production requires finding suitable expression hosts. An overview of Food and Drug Administration-approved ADCs currently on the market is given by Tong et al. The resulting products are known as antibody-drug conjugates (ADCs) and extend the usage of immunotherapeutics. It is frequently employed for the incorporation of uAAs into monoclonal antibodies and antibody formats, predominantly of the IgG1 subclass, to enable site-specific click reactions with small molecule payloads. Altogether, the codon reassignment by which an uAA is site-specifically incorporated instead of a canonical amino acid is called the expansion of the genetic code. The most common approach to achieving orthogonality is using a tRNA and its cognate RS from an organism phylogenetically distant from the expression host. In other words, the technology used and the uAA must be orthogonal in the host cell. The uAA to be site-specifically incorporated must not be recognized by endogenous tRNAs and tRNA synthetases (RSs). Several conditions need to be fulfilled to successfully incorporate an uAA at the desired position in a target protein. These desirable features have opened doors to various applications and manipulations of endogenous and heterologous proteins. Such reactions are bioorthogonal and, frequently, they proceed in one step, without the formation of side products. Particularly after the formulation of the official definition of “click” chemistry, research has focused on the incorporation of unnatural amino acids (uAAs) into proteins with reactive groups that cannot be found in nature and react with biological systems. In the past two decades, the demand for site-specific incorporation of reactive chemical groups in a wide range of proteins has been growing to gain novel properties. pastoris in the development of antibodies for subsequent conjugations with, e.g., bioactive payloads. Applying the results of this work in glycoengineered strains, and taking further steps in process development opens great possibilities for utilizing P. Pichia pastoris was successfully employed for cost-effective laboratory-scale production of a monoclonal antibody with an unnatural amino acid. Successful site-specific incorporation of pAzF was confirmed by mass spectrometry.
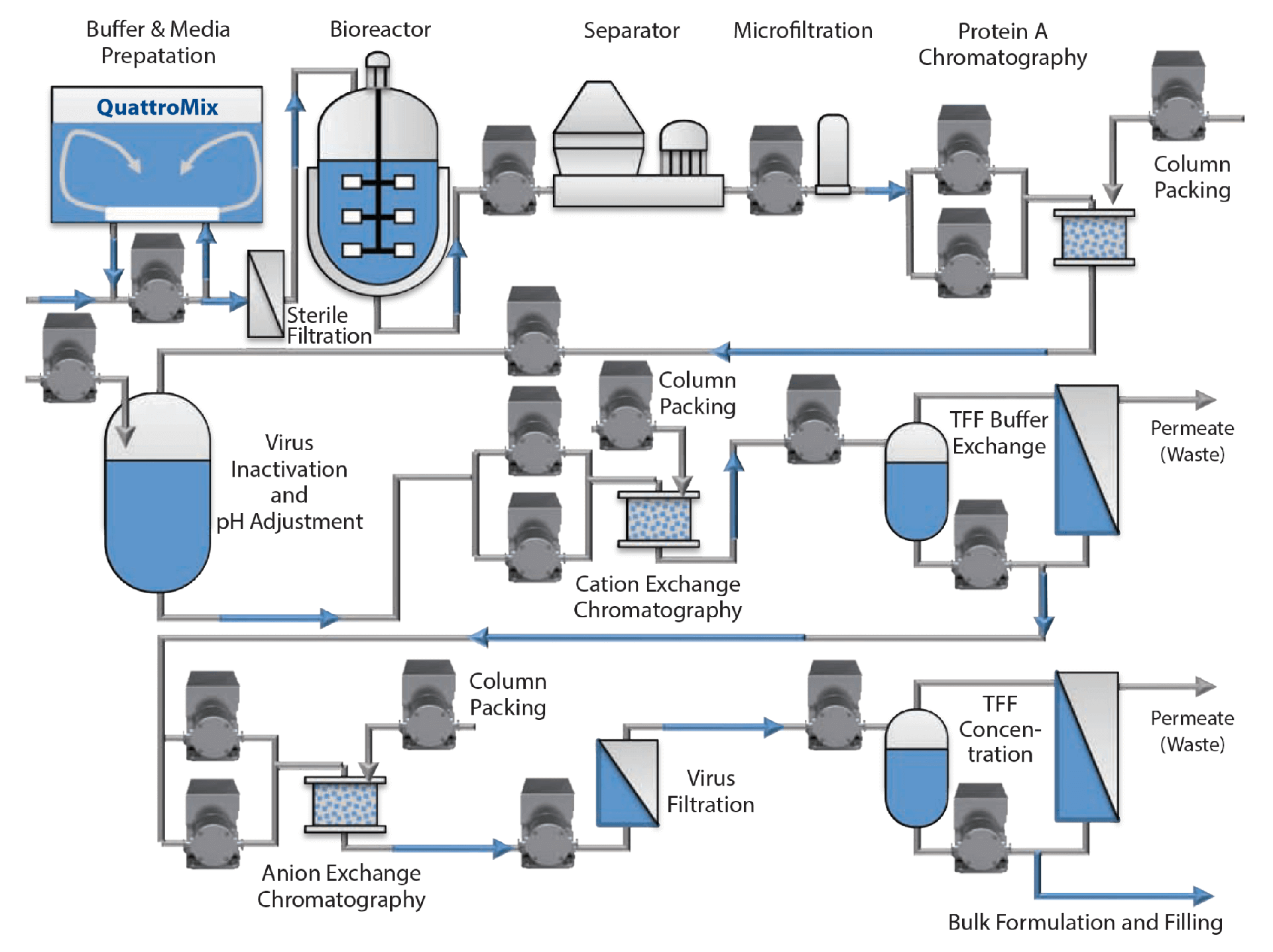
pastoris strains increased IgG and IgG pAzF productivity by around 50- and 20-fold compared to screenings, yielding up to 238 mg L −1 and 15 mg L −1 of fully assembled tetrameric protein, respectively. Fed-batch bioreactor cultivations of engineered P. In the case of Fab, a knockout of vacuolar targeting for protein degradation further increased protein yield. ResultsĬo-translational transport of proteins into the endoplasmic reticulum through secretion signal prepeptide change and overexpression of lumenal chaperones Kar2p and Lhs1p improved the production of trastuzumab IgG and its Fab fragment with incorporated pAzF.
#STEPS IN ANTIBODY PRODUCTION CODE#
Combining the knowledge of protein folding and secretion with bioreactor cultivations, the aim of the work was to make the production of monoclonal antibodies with an expanded genetic code cost-effective on a laboratory scale. This approach was applied for the production of trastuzumab IgG carrying p-azido- l-phenylalanine (pAzF) in the industrial yeast Pichia pastoris. It involves reassignment of a codon to another, e.g., unnatural, amino acid and requires the action of a pair of orthogonal tRNA and aminoacyl tRNA synthetase modified to recognize only the desired amino acid. Expansion of the genetic code is a frequently employed approach for the modification of recombinant protein properties.


 0 kommentar(er)
0 kommentar(er)
